This project will leverage two sets of existing cohort infrastructures, the first to generate new multi’omic data in an experimental design targeted to a deep understanding of IBD, and the second to rapidly build our data sharing interface around one of the only extant pilot multi’omic profiles of IBD. First, our three Sample Generation components (PRISM, RISK, and MLI) are led by consortium members from the CCFA to collect new microbiome samples. These will profile new-onset and established pediatric and adult IBD using a uniform protocol and an experimental design specific to deep longitudinal multi’omic data generation. Second, a fourth Data Generation component will take advantage of a pair of additional cohorts (two Swedish cohorts) from which one of the only existing multi’omic datasets has been generated in IBD, allowing us to populate and pilot our bioinformatic infrastructure in parallel to generation of a complete, feature-rich multi’omic data resource.
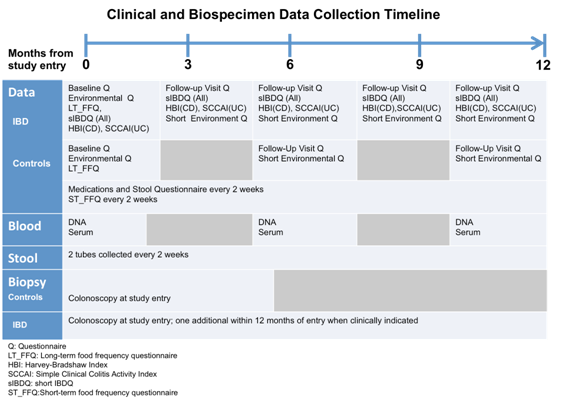










The Prospective Registry in IBD Study at MGH (PRISM) is a referral center-based, prospective cohort of IBD patients initiated in 2005 with ongoing recruitment enrolling patients from within the physician practices at the Crohn's and Colitis Center at Massachusetts General Hospital. The goal of PRISM is to establish a well-phenotyped cohort of patients with inflammatory bowel disease with corresponding biologic samples including blood, stool and biopsies to help identify potential biomarkers that can predict a patient's response to drugs and toxicity, to accurately define disease activity, and to predict future relapse and prognosis. To date, there are 1,983 patients over the age of 18 enrolled with a diagnosis of UC, CD, or indeterminate colitis in PRISM. Blood samples are collected at entry; stool and biopsies are obtained upon further consent from the patient. From this cohort, we will recruit 24 adult subjects with newly-diagnosed, treatment-naive IBD and 6 age-matched healthy controls.
The Pediatric Inception Cohort emerges from the CCFA-sponsored RISK study, which started in 2009 involving 28 pediatric centers and completed recruitment in 2013. When a child is suspected to have IBD and about to get a colonoscopic examination, the family is approached and enrolled prior to therapy. To date, roughly 1,130 CD, 139 UC, 129 IBDU, and 380 non-IBD subjects have been recruited to RISK. The biosampling pipeline is well-established at Emory University and Cincinnati Children’s Hospital as a result of their involvement in RISK allowing for streamlined recruitment and collection of repeated samples. From this cohort, we will recruit 24 pediatric subjects with newly-diagnosed, treatment-naive IBD and 6 age-matched healthy controls.
This cohort has been formed to test the hypothesis that colonic microbiota of the mucosal-luminal interface (MLI) form a mosaic of functional communities, organized by interactive microbial and host products and resultant microbial composition started in 2007. Centered at the Cedars-Sinai (C-S) IBD Center, patients undergoing clinically-indicated endoscopy are recruited to fulfill normal and IBD cohorts with full genomic, serology and clinical phenotyping. Endoscopic lavage is used to sample the MLI at 2 segments, and additional biospecimens (stool, biopsy, serum, urine, saliva) are available. Over 180 patients and 300 samples have been collected to date with well-established biosampling protocols and clinical coordinators in place to recruit and collect repeat samples throughout the course of the year. From this cohort, we will recruit 24 adult patients with established IBD diagnosed greater than 5 years from the time of enrollment and 6 age-matched healthy controls.
Existing multi’omic data from two Swedish studies, the Swedish Twin and Swedish Longitudinal cohorts, will be used by the IBDMDB as a ready-made “proof-of-principle” dataset around which we will organize and prototype our computational infrastructure in parallel with primary data generation. For the Swedish Twin IBD cohort, twin pairs of the same sex, 18 years or older were identified and a questionnaire was sent to all twins including questions about diagnosis of IBD and general gastrointestinal symptoms. Zygosity was determined using the method applied by the Swedish twin registry, relying on childhood resemblance.
The Swedish Longitudinal study includes representation of both genders (56 males and 63 females). The subjects provided fecal samples at 3-month intervals for a maximum of 10 points. Women (but no children) were included in the study group, but not if they were pregnant or breastfeeding. The disease phenotype and extent was determined using the same criteria as described above for the Swedish twin cohort. Additional data collected from each study include year of diagnosis, close relatives with IBD, smoking status, primary sclerosing cholangitis, clinical status at diagnosis, perianala fistules, maximal spread, date of colonoscopy, resection, calprotectin levels, antibiotic usage (12mos), NSAID usage (12mos), gastroenteritis (3mos), abdominal pain, bowel movements, diarrhea, blood or mucus in stool, and prescribed treatments.